
When summer comes, you can eat again.

However, one netizen complained bitterly: “In summer, my mother won’t even drink a bottle of carbonated drinks.”
The story goes like this…
▼
Although my mother was well-intentioned, she was cheated by rumors and ate according to this watch for a long time. I was really afraid that the whole family would suffer from malnutrition.
[Acidic constitution] and [alkaline constitution] do not exist at all.
There are no research articles based on [acidic constitution] or [alkaline constitution] in the nutrition and medical circles at home and abroad, and no relevant articles can be found in various authoritative literature databases.
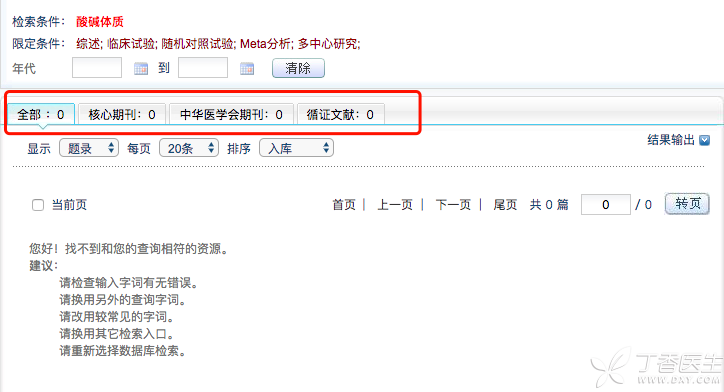
The fact is obvious, because [acid and alkali constitution] does not exist at all. But why is there such a heated discussion among friends?
Because what the folk call “acidic constitution” is actually a change of concept with “acidosis”, such as ketoacidosis of diabetics.
Acidosis occurs only after taking a certain medicine or suffering from a certain disease, [1] rather than [acidic constitution can lead to diseases], just like sleeping because you are sleepy, but it is absurd to say that sleeping is sleepy because you are sleepy, which is a typical inversion of cause and effect.
[Alkaline Constitution] Healthier is totally nonsense. The PH value of normal people’s blood is 7.3 ~ 7.4, which is weakly alkaline. The human body has a strong buffer system to ensure the stability of PH value in blood. [2]
What’s more, the human body is originally a state of acid and alkali coexistence. For example, the stomach, skin and vagina are acidic and the intestinal tract is alkaline. There is no single acidic or alkaline constitution.
The acidity and alkalinity of food can be ignored.
Food does have acid-base properties in food chemistry, and the classification method is determined according to the chemical properties of [ash] obtained after food combustion. [3]
[Ash] If it contains more phosphorus, sulfur and chlorine, it will be acidic when dissolved in water and belongs to acidic food. If there is more potassium, sodium, calcium and magnesium in [ash], it is alkaline and belongs to alkaline food, [3] and the taste of the food itself are not directly related.
For example, most vegetables and fruits are alkaline, while most meat and cereals are acidic, but this acid-base classification in food chemistry is only used to distinguish the chemical composition of food.

After complicated digestion and absorption in human body, food will form acidic, alkaline and neutral metabolites, which are completely different from the ash content of food burned in vitro. [4]
Generally speaking, any food you eat will become acidic under the action of gastric juice after entering the stomach, and will become alkaline in the intestinal tract.
Take the most common carbonated beverages for example, carbonated beverages are [acidic] because carbon dioxide dissolves in water.
However, some of the carbon dioxide in carbonated beverages will escape when the pop can is opened, and most of it will be discharged out of the body with [hiccups] after drinking, so no large amount of carbon dioxide will be absorbed by the human body, thus affecting the acid-base balance.

For a bottle of 3-5 litres of carbonated beverage, it is estimated that the intake of carbon dioxide when drinking is between 0.5 and 1.5 litres, [5] From this calculation, the intake of carbon dioxide after drinking a bottle of 330 milliliters of carbonated beverage will not exceed 0.5 litres, and very few of them actually enter the blood.
In fact, part of the product of human metabolism is carbon dioxide. This carbon dioxide is either discharged from the body or combined with water in the body to form carbonic acid, which makes a large amount of carbon dioxide enter and leave the body with respiration.
Each person has about 0.3 liters of carbon dioxide every minute from the blood into alveoli and then exhaled out of the body. A person produces about 432 liters of carbon dioxide every day. [6]

In other words, carbon dioxide has a much greater effect on blood acidity during breathing than drinking a bottle of carbonated beverage.
Moreover, carbon dioxide, as a food additive recognized by the state, has strict national standards. Reasonable use will not cause harm to the body. [7] It is diseases and drugs that really affect the pH imbalance of the body.
Instead of paying attention to [acid and alkalinity], it is better to pay attention to [diversity]
The choice of healthy food is not based on their [acid and alkali], but on their own nutritional value.
It is more important to match food reasonably to ensure the diversity of food and balanced diet. Stop arranging recipes according to the acid and alkali table of food!
Again, the acidity and alkalinity of food are almost negligible compared with the alkaline environment in gastric acid and intestinal tract, and naturally will not affect the acidity and alkalinity of human blood.
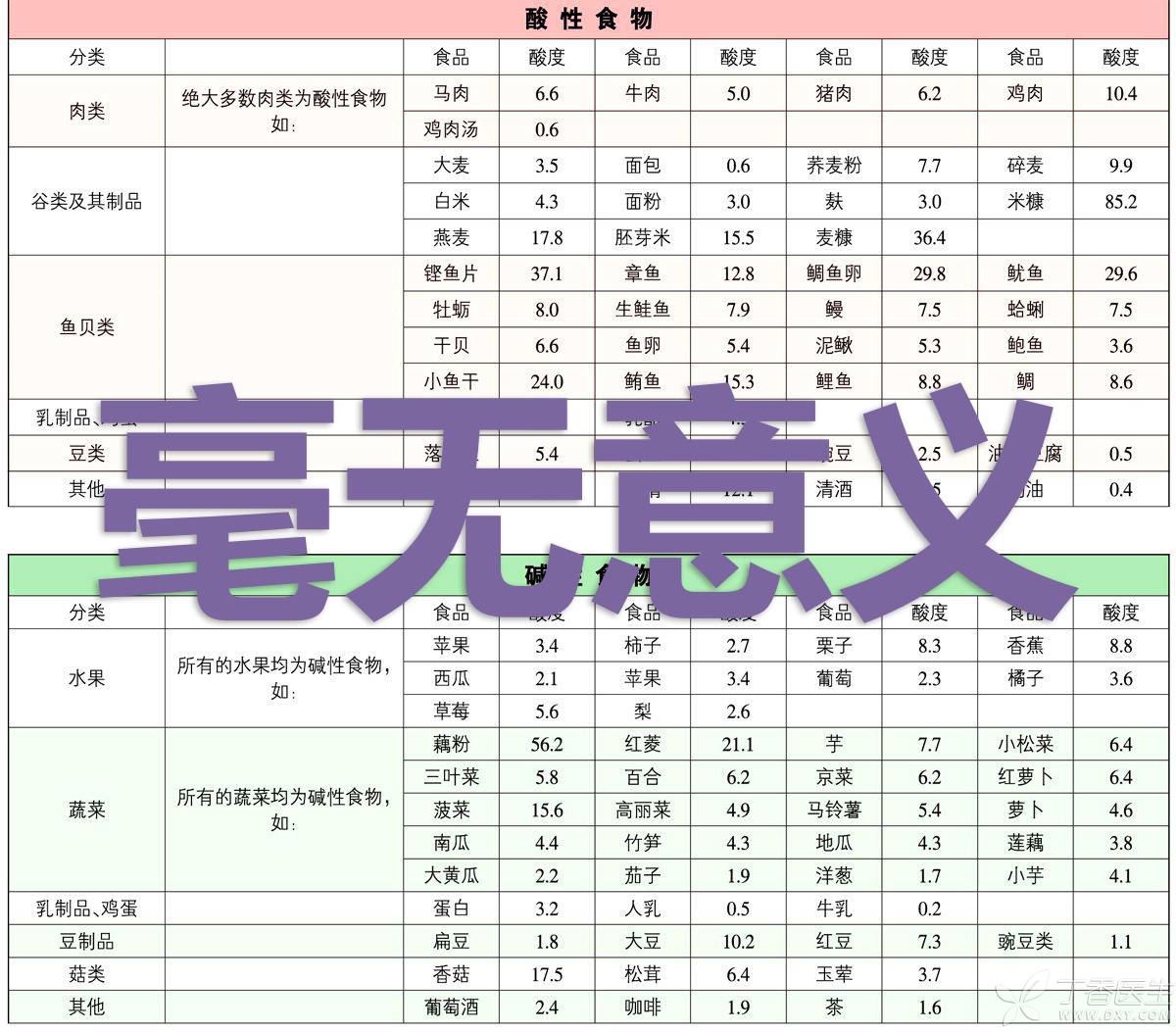
That is to say, this so-called acid and alkali food table, which is widely circulated on the Internet…
Besides, how can summer be a complete summer without watermelon and ice soda?
